To find a disease-modifying treatment for Parkinson disease, we first need to understand what goes awry at a cellular level. We use a combination of cell biology, biochemistry, and physiology techniques to tackle this question while translating discoveries into therapeutic development.
We are particularly interested in the role of palmitoylation in regulating the biology of alpha-synuclein, a key protein involved in Parkinson disease, Lewy body dementia, and other “synucleinopathies”.
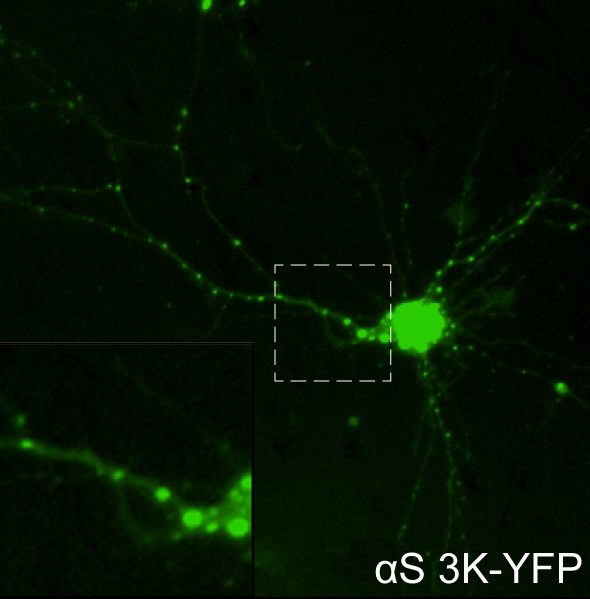
Modulation of alpha-synuclein pathology by palmitoylation. In disease, alpha-synuclein (αS) disrupts vesicle trafficking in the neuronal cell body and pre-synaptic compartment. In contrast, protein palmitoylation (lipid modification) of various membrane-associated proteins is required for normal trafficking. We have found that enhancing palmitoylation by inhibiting the depalmitoylase APT1 reduces αS-mediated toxicity and inclusions in cultured neurons. In collaboration with our neighbors in the Silke Nuber lab, we demonstrated that PD model mice treated with an APT1 inhibitor, ML348, also exhibit improved PD-like symptoms and reduced αS pathology. We are now using proteomic approaches, followed by mechanistic investigations, to characterize the substrates and pathways altered by these interventions in our efforts to translate our findings towards novel therapeutics.

Palmitoylation of specific PD-associated risk genes. Numerous genetic risk factors for PD have been identified, but we don’t understand their mechanistic role. A subset of these genes encode for vesicle proteins with potential palmitoylation sites. We would like to understand how palmitoylation affects their function in trafficking in the context of PD. For example, we have discovered that the product of the PD risk gene SYT11 (synaptotagmin-11) is palmitoylated, and this modification is crucial for its stability and role in modulating αS multimers.

Alpha-synuclein (αS) and synaptic function. Disease-associated αS has complex effects on synaptic vesicle exocytosis and recycling. We have developed methods to measure these dynamics in human induced pluripotent stem cell-derived neurons from PD patients. These techniques will enable us to obtain molecular-level insights into αS-induced synaptic dysfunction in a highly disease-relevant context.

Abcam tower. Currently on hold due to lack of necessary materials.